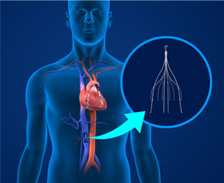
IVC (inferior vena cava) filters are cage like, small metal devices that are implanted in patients’ veins in order to stop blood clots from reaching their lungs. Unfortunately, these devices have now been linked to a number of serious, sometimes fatal, complications. These include organ perforation, breakage and migration of the IVC filter.
What Is an IVC Filter?
Basically, it is a type of retrievable device that catches a blood clot before it is able to travel to the lungs. It looks very much like a metal cage and it is inserted in the vein through surgery. Usually, patients at risk of blood clots will take blood thinners, but some patients are not able to do so for medical reasons. In this case, an IVC may be prescribed.
The first IVC was used in 1979. Since then, it has been used more and more frequently. It is believed that some 259,000 IVC filters were inserted into patients by 2012.
IVC Filters and Blood Clots
Blood clots can develop in different parts of the body. DVT (deep vein thrombosis), however, can occur in the upper and lower extremities and deep inside the pelvis. In most cases, a DVT is not life threatening in itself. A problem occurs, however, if the clot travels to the lungs, where it can cut off normal blood flow. This is often a fatal condition. A patient who has a DVT that has traveled to the lungs has what is known as a PE (pulmonary embolism). Some 300,000 people die from PEs every year, with it actually being the third most common cause of death in patients who are in hospitals.
Many times, an IVC filter will work really well and is able to stave off a blood clot that is traveling. At other times, however, the filter will start to migrate away from where it was inserted, at which point in becomes ineffective. It is also possible for the device to puncture the vein into which it was inserted, which can lead to bleeding and various other significant complications. Surgeons insert the IVC filter because they intend to do good but sometimes, things don’t work out that way.
How Do IVC Filters Work?
The largest vein in the human body is the inferior vena cava (IVC). Through the IVC, de-oxygenated blood is moved from the lower legs all the way to the heart and then lungs. IVC filters are placed within this vein to stop a clot from traveling upwards. This is done through a thin tube, or catheter, which is inserted into the inferior vena cava. A small incision is made in the groin or neck to reach the vein. The metal wires on the device capture blood clots and then trap them, stopping them from reaching the lungs.
There are a few permanent IVC filters, but most are temporary and retrievable. The retrieval of the filter is done in virtually the same way as the implantation. Usually, some x-ray or contrast dye is injected around the filter so that doctors can see whether removal can be safely completed. A snare, similar to a catheter, is then inserted, which grabs the hook on the end of the filter. It then goes into a sheath and is pulled out.
Different Types of IVC Filters
There are two kinds of IVC filters, namely, the permanent filter and the optional/retrievable filter. A retrievable filter only gives short term protection. It should be removed as soon as the risk for PE is gone. Unfortunately, this filter can cause significant complications, particularly when it migrates. These complications include organ and blood vessel perforation.
Some people are classed as being a good candidate for an IVC filter. These include:
- People who continue to have DVTs even if they are taking anticoagulation medication to help thin the blood.
- People who have an allergy or other adverse reaction to anticoagulation medication. These include those who have recently experienced surgery, trauma or bleeding.
Complications with Retrievable IVC Filters
A number of possible risks are associated with retrievable IVC filters, including failing to stop clots in their tracks and damaging the veins. There are a number of serious complications as well, which tend to be caused when the filter breaks away or even breaks apart and starts to travel to a different part of the body.
An official safety alert was released by the U.S. Food and Drug Administration (FDA) in 2010 in relation to retrievable filters. This was after 921 individual reports had been received from patients who had IVC filters between 2005 and 2010. Some of the complaints they received included:
- Filter migration
- Filter perforation
- Filter fracture
- Device embolization, where different components detach from the device
Thirty-five percent of the complaints were about filter migration. The second most common complaint was embolization.
Another concern noted by the FDA is that retrievable filters would be left in place long after the PE risk had gone. In 2014, a new safety communication was released to state that all filters should be removed between 29 and 54 days after implantation, if the PE had subsided.
Studies Confirming Warnings
A number of studies have confirmed that there are indeed problems with IVC filters. The most significant was the 2013 study by Journal of the American Medical Association (JAMA) – just 58 of 679 filters were removed. In terms of the filters that stayed in the body, attempts to removed it failed in 18.3%. Specifically, 8 people had embedded devices, 3 people had devices that were protruding through their vessels, 2 people noted their device had migrated into an abnormal position, and 1 person had a blood clot within the filter. Venous thrombotic events occurred in 7.8% of cases. 25 patients suffered a PE.
It was also found that five specific IVC filters were more prone to failure. These were:
Bard’s Recovery was developed in 2003 and was updated to the G2 in 2005. Twenty-five percent of the Recovery filters fractured or broke apart. One of the patients died at home, although the cause of death has not been revealed. The G2 had a 12% failure rate. It was updated by the G2 Express in 2008, and this also had a 12% failure rate.
Cook’s filters, both the Gunther Tulip and the Celect, had a history of perforating the wall of the veins. This often occurred 71 days after implantation. In 40% of cases, the filter migrated.
Lawsuits
Those who have had complications as a result of the IVC filters have filed numerous lawsuits, both individually and in class action. A number of specific allegations have been made, including:
- Failure to warn
- Negligence
- Manufacturing defects
- Design defects
- Negligent misrepresentation
- Breach of implied warranty
The first lawsuits were filed in 2012 in California. All running lawsuits, of which there were over 100, were consolidated in 2014. A settlement was reached with individual plaintiffs in 2015. Further lawsuits were consolidated that same year.
Life Threatening Complications
Filters have been designed specifically to help save people’s lives, as they hope to prevent a PE. Yet, their side effects can be equally dangerous. In the 2010 FDA report, over 900 adverse events were listed in relation to various IVC filters. These include:
- 70 cases of filter perforations
- 328 cases of device migrations
- 56 cases of filter fractures
- 146 cases of embolisms
As soon as Bard started to receive complaints about their Recovery filter, they hired a professional, Dr. John Lehmann, to look into the case. His findings were that further investigation would be needed. It also appeared that Bard was aware of the problem, but that they did not inform the FDA or the general public. The report by Dr. Lehmann was circulated among employees, who were told it needed to remain secret. However, the report had to become public when some of the cases went to court but Bard continues to argue that they should remain confidential. Unfortunately for Bard, one lawyer accidentally disclosed the information in 2012. A request was then put in to destroy all copies of the report, but not all courts agreed to this.
In 2015, an investigation by NBC News alleged that Bard knew about the malfunctions in their device before the Lehmann report. They have suggested that FDA clearance was denied in 2002, and that Bard then hired Kay Fuller in order to receive clearance. Fuller, allegedly, had concerns about the device and did not agree to request clearance for it. Yet, her name and signature is found on the clearance application to the FDA. Fuller claims she did not sign it and that it has been forged in her name.
Additional Resources:
- Complications of Inferior Vena Caval Filters
- Cleveland Clinic. (2014). Inferior vene cava (IVC) filter retrieval
- Prevalence of fracture and fragment embolization of bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. JAMA Internal Medicine
- Indications, complications, and management of inferior vena cava filters
- University of Michigan. (2013). Inferior vena cava (IVC) filters
- U.S. Food and Drug Administration. (2010, August 9). Removing retrievable inferior vena cava filters: Initial communication
- U.S. Food and Drug Administration. (2014, May 6). Removing retrievable inferior vena cava filters: FDA safety communication
- Fractured Bard Recovery, G2, and G2 express inferior vena cava filters: incidence, clinical consequences, and outcomes of removal attempts